40 energy diagram for a two step reaction
Answered: Draw an energy diagram for a two-step… | bartleby Draw an energy diagram for a two-step reaction, A → B → C, where the relative energy of these compounds is C < A < B, and the conversion of B → C is rate-determining. check_circle Expert Answer Want to see the step-by-step answer? See Answer Check out a sample Q&A here. Want to see this answer and more? Energy Diagrams of Two Step Reactions - YouTube Energy Diagrams of Two Step Reactions. 2,095 views Jan 30, 2018 Watch Complete videos @ Organic Chemistry 1 ...
Krebs cycle / Citric acid cycle / TCA Cycle with steps and ... Mar 10, 2022 · The third step of the citric acid cycle is the first of the four oxidation-reduction reactions in this cycle. Isocitrate is oxidatively decarboxylated to form a five-carbon compound, α-ketoglutarate catalyzed by the enzyme isocitrate dehydrogenase. This reaction, like the second reaction, is a two-step reaction.
Energy diagram for a two step reaction
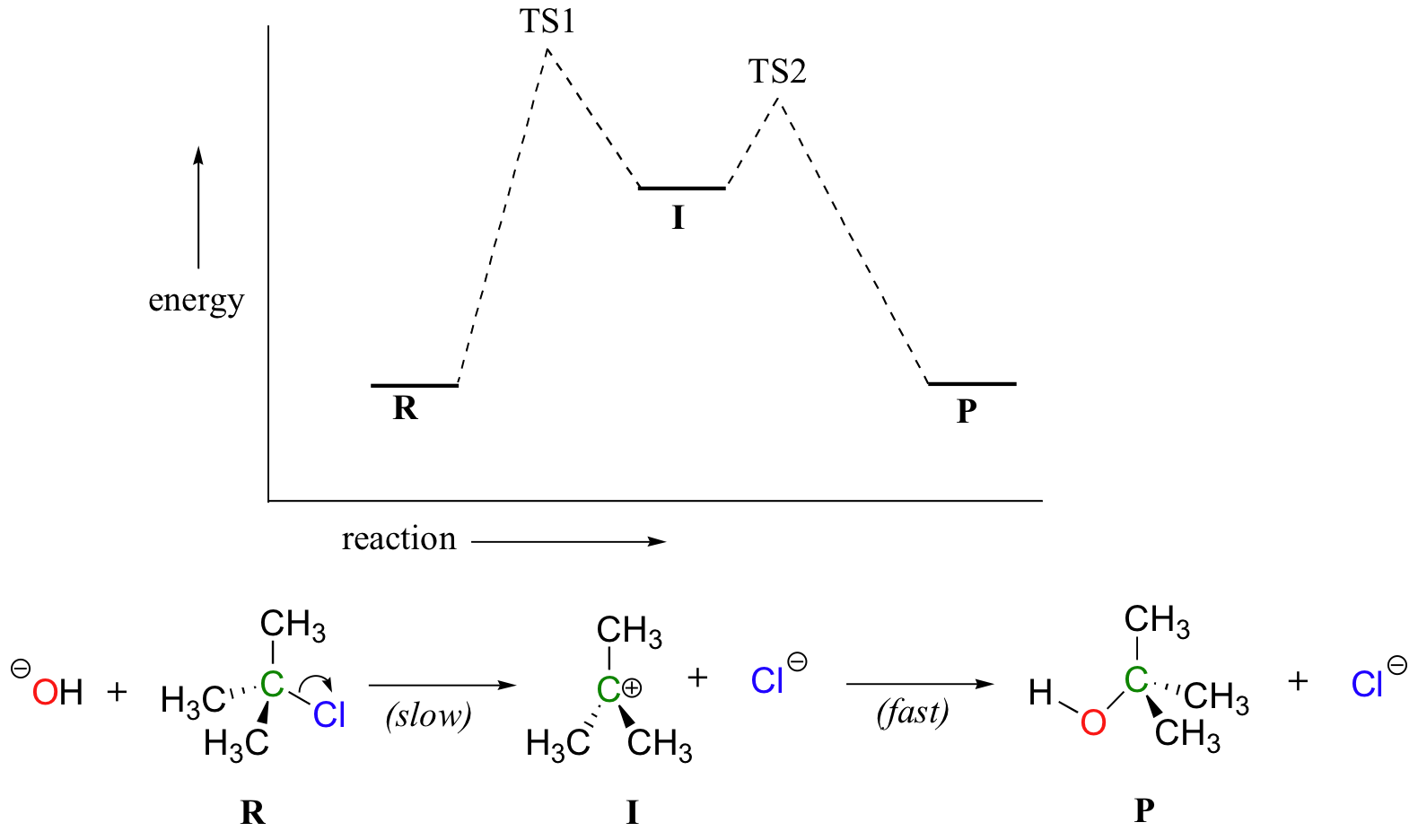
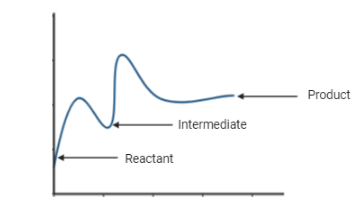
7.4 SN1 Reaction Mechanisms, Energy Diagram and Stereochemistry Figure 7.4a Energy diagram for SN1 reaction between (CH3)3CBr and H2O. The connection between the first two curves represent the carbocation intermediate. Generally, the intermediate is the product of one step of a reaction and the reactant for the next step. The intermediate is at a relatively lower energy level compared to the transition ... The reaction-energy diagram for a two step endothermic reaction with a ... The reaction-energy diagram for a two step endothermic reaction with a rate-limiting second step is to be drawn. Concept introduction: The graphical representation of chemical reaction in which x-axis represents energy of the reaction and y-axis represents the extent of reaction process is called energy profile diagram. Draw an energy diagram for a two-step reaction, $$ A \righ | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for a two-step reaction, $$ A \rightarrow B \rightarrow C, $$ where the relative energy of these compounds is C < A < B, and the conversion of $$ B \rightarrow C $$ is rate-determining..
Energy diagram for a two step reaction. Potential Energy Diagrams - Chemistry - Catalyst, Endothermic ... - YouTube This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... 6.8: Energy Diagram for a Two-Step Reaction Mechanism A potential energy diagram for an S N 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition states. Exercise. Draw structures representing TS1 and TS2 in the reaction above. Solved Label the energy diagram for a two-step reaction. | Chegg.com Question: Label the energy diagram for a two-step reaction. This problem has been solved! See the answer ... Answered: Draw a reaction-energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. arrow_forward. Sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic.
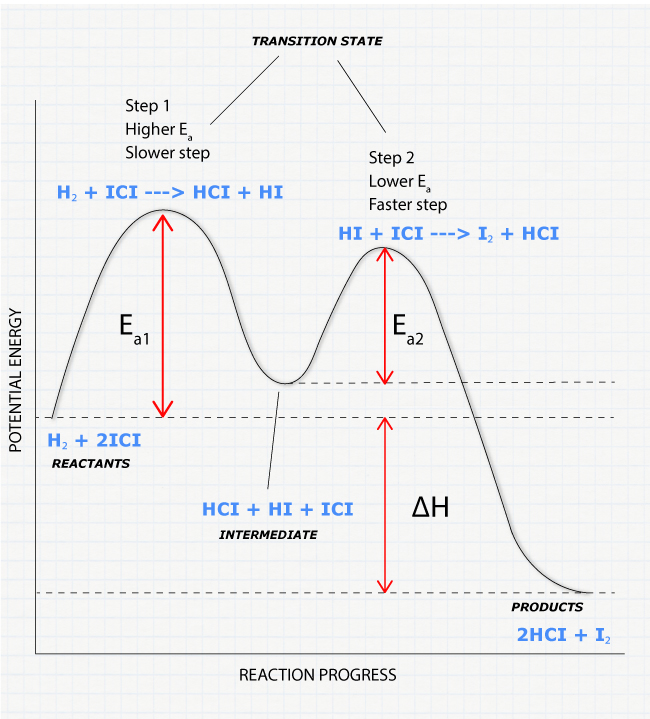
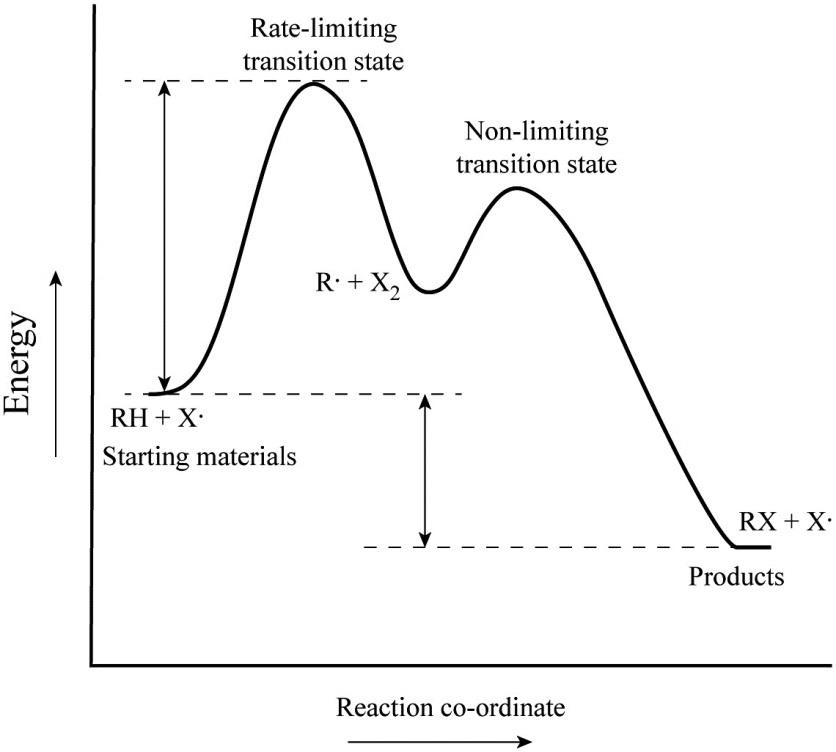
How to Calculate Bond Energy: 12 Steps (with Pictures ... Mar 29, 2022 · The final step to calculating bond energy is to determine whether the reaction releases energy or consumes energy. An endothermic (one that consumes energy) will have a final bond energy that is positive, while an exothermic reaction (one that releases energy) will have a negative bond energy. Solved Label the energy diagram for a two-step reaction. - Chegg Expert Answer. 100% (54 ratings) Transcribed image text: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate. Reaction Mechanisms and Potential Energy Diagrams | CK-12 ... The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled ... Question #62891 | Socratic In the figure, the second step has a larger Eact and is therefore the rate determining step. So, to draw a two step exothermic reaction, with the second step rate determining, keep two things in mind: 1) Eact of the second step must be larger than Eact of the first step. 2) Energy of products must be lower than energy of reactants. Answer link.
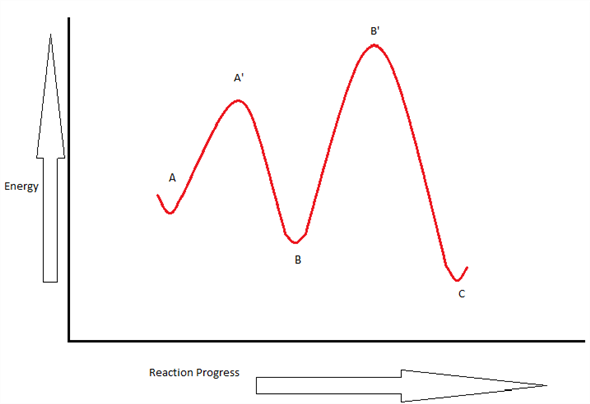
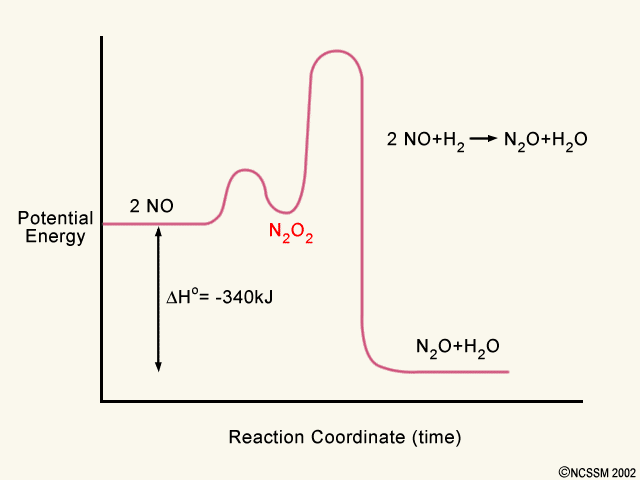
Draw an energy diagram for a two-step exergonic reaction ... - Numerade in this question. We're going to draw the energy during um for two step reaction where the second step of the reaction is faster than the first step of the reaction. It's no. Let's recall the definition of Gibbs free energy change and that is the difference between the free energy of the products. My enough see free energy of the reactant. It's so yeah, the gifts rich free energy change is ... 12.7 Catalysis - Chemistry 2e | OpenStax The catalyzed reaction is the one with lesser activation energy, in this case represented by diagram b. Check Your Learning Reaction diagrams for a chemical process with and without a catalyst are shown below. Both reactions involve a two-step mechanism with a rate-determining first step. Label the energy diagram for a two-step reaction - Learn CBSE Forum Label the energy diagram for a two-step reaction. ... An energy profile diagram is a theoretical representation that shows how the energy of the ... PDF Potential energy diagram for a two-step sequential reaction Potential energy diagram for a two-step sequential reaction A 1 19 3 50 AB* 4 33 3 40 B 3 28 3 30 BC* 4 41 3 20 C 5 13 3 10 3 0 the red lines are error bars on the x -value Adjust energies Derived from Scott Sinex's Excel chart 1 2 3 4 5 0 10 20 30 40 50 Energy Reaction coordinate step 1 step 2 A B C A B C k 1 k 2 B (point 3) is an intermediate
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
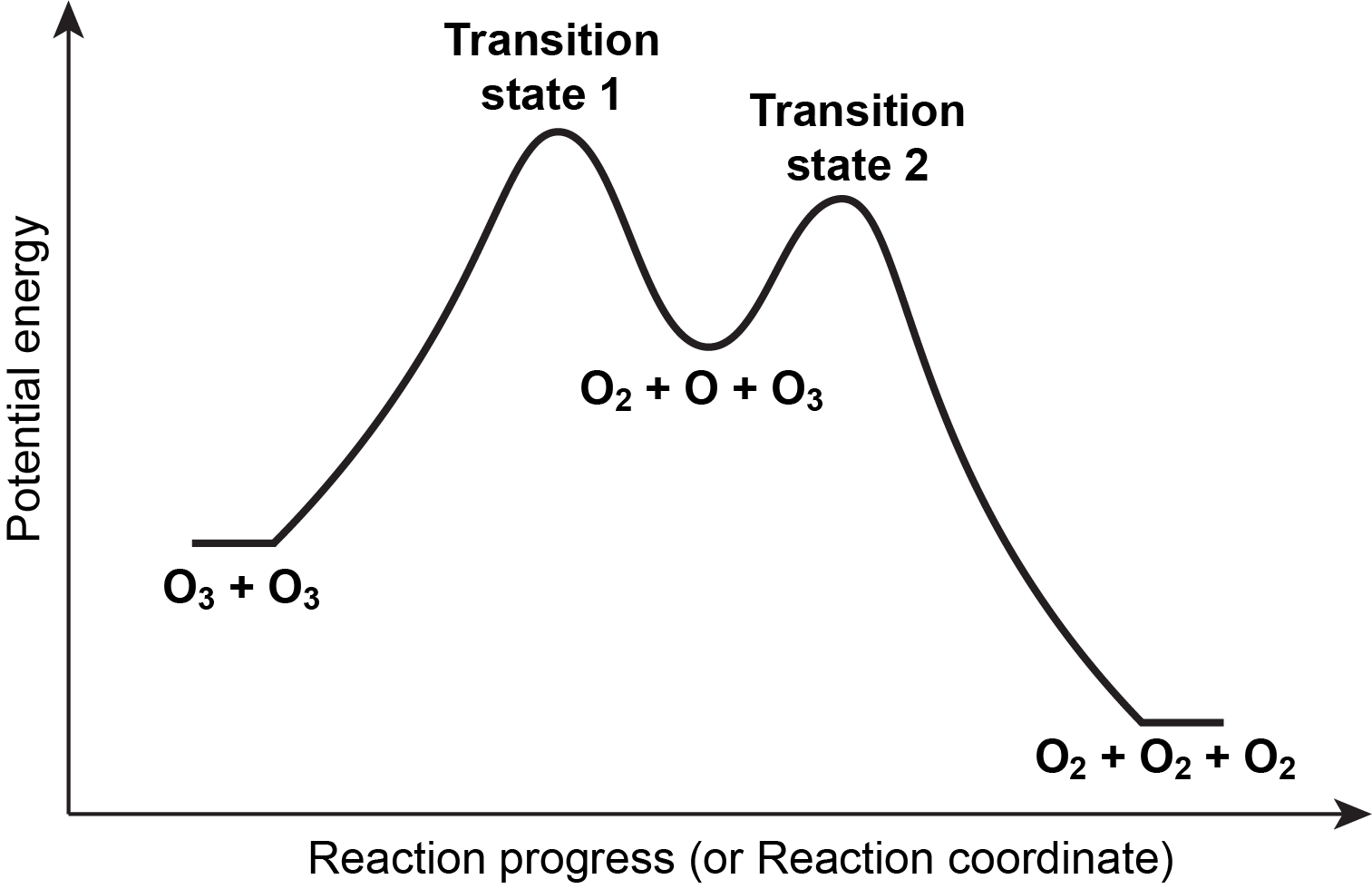
The role of adsorbed hydroxide in hydrogen evolution reaction ... Oct 19, 2020 · b, Reaction energy diagram to illustrate the HER reaction mechanism. On the too-weak binding side of the OH* volcano, the rate is limited by water dissociation (the Volmer step).
Multistep reaction energy profiles (video) | Khan Academy Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction.
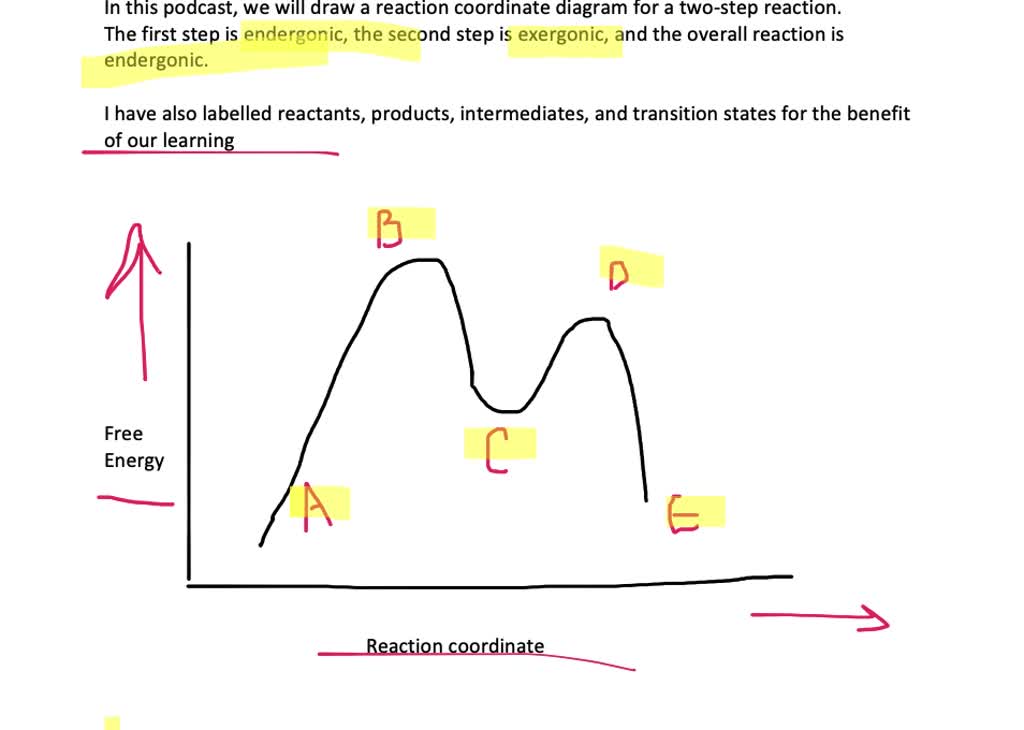
draw a reaction coordinate diagram for a two step reaction in which the first step is endergonic t 3
Rate-determining step - Wikipedia In a multistep reaction, the rate-determining step does not necessarily correspond to the highest Gibbs energy on the reaction coordinate diagram. [8] [6] If there is a reaction intermediate whose energy is lower than the initial reactants, then the activation energy needed to pass through any subsequent transition state depends on the Gibbs ...
Draw a reaction coordinate diagram for a two-step reaction i - Quizlet Draw an energy diagram of a reaction with the following characteristics: (a) A one-step reaction with a negative Δ G \\Delta G Δ G (b) A one-step reaction with a positive Δ G \\Delta G Δ G (c) A two-step reaction with an overall negative Δ G \\Delta G Δ G, where the intermediate is higher in energy than the reactants and the first transition state is higher in energy than the second transition state
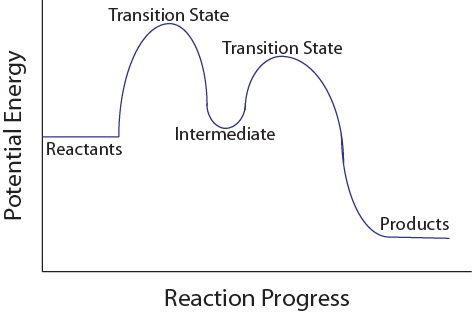
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
Answered: Draw a reaction energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies.
Cellular Respiration Equation, Types, Stages, Products & Diagrams Dec 07, 2021 · As shown in the above diagram, glycolysis takes place in the cytosol. Glycolysis is referred to as a “ten enzyme-catalyzed reaction” but the overall simplified equation is: C 6 H 12 O 6 + 2 NAD+ + 2 ADP + 2 P → 2 pyruvic acid, (CH 3 (C=O)COOH + 2 ATP + 2 NADH + 2 H+
Chemical Equation for Endothermic Reaction | Equation Balancer Jun 28, 2021 · Similarly, the potential energy diagram can also help you to predict the nature of the reaction. We hope this article helped you learn about endothermic reactions and exothermic reactions. You can also find step by step guides for balancing chemical equations here. Or learn how to find percent yield of a chemical reaction. Frequently Asked ...
Draw an energy diagram for each reaction. Label the axes, th - Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, $$ \Delta H ^ { \circ } , \text { and } E _ { \mathrm { a } }. $$ a. A concerted, exothermic reaction with a low energy of activation. b. A one-step endothermic reaction with a high energy of ...
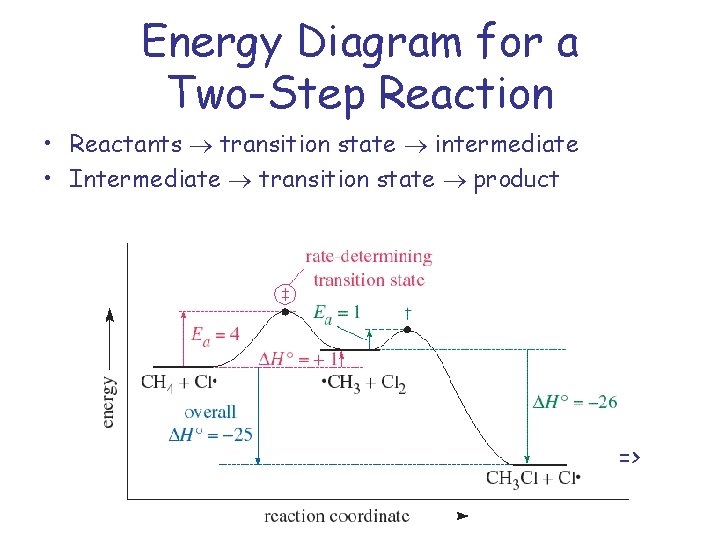
Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall.
SOLVED:Draw a reaction coordinate diagram for a two-step ... - Numerade Sketch an energy diagram for a two-step reaction in which both steps are exe… 06:56. Draw an energy diagram for a two-step exergonic reaction whose second step i… 02:12. Draw a graph showing the reaction pathway for an overall exothermic reaction… 01:07. A chemical reaction is endothermic and has an activation energy ...
Mechanisms and Potential Energy Diagrams - Course Hero A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above.
Energy - Wikipedia The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the components of an object – and potential energy reflects the potential of an object to have motion, and generally is a function of the position of an object ...
Solved: Sketch an energy diagram for a two-step reaction in which ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G‡, and overall Δ G °. Step-by-step solution 91% (11 ratings) for this solution Step 1 of 3
Solved Choose the energy diagram for a two-step reaction, A - Chegg Choose the energy diagram for a two-step reaction, A → B → C, in which the relative energy of the compounds is A< C < B, and the step A→ B is rate-determining. Select the single best answer. Reaction coordinate Reaction coordinate IV.
6.15: Energy Diagram for a Two-Step Reaction Mechanism A potential energy diagram for an S N 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition states. Exercise. Draw structures representing TS1 and TS2 in the reaction above.
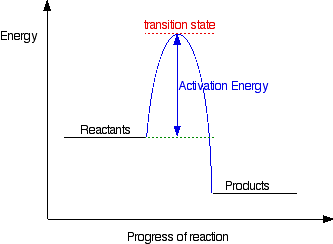
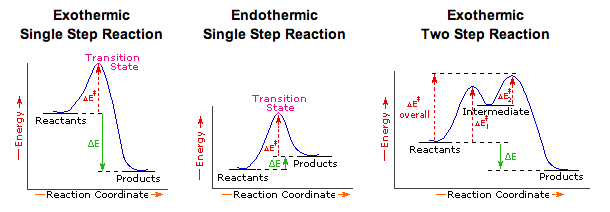
Energy Diagrams of Reactions | Fiveable The energy diagrams below show what should be known for the test. Before looking at the specifics of each, you should be aware of a few terms: Activation Energy - Energy necessary for the reaction to occur. Activated Complex - The maximum point of energy, where the reactants turn into the products and the reaction occurs.
Draw an energy diagram for a two-step reaction, $$ A \righ | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for a two-step reaction, $$ A \rightarrow B \rightarrow C, $$ where the relative energy of these compounds is C < A < B, and the conversion of $$ B \rightarrow C $$ is rate-determining..

How to Draw Multi-Steps Energy Profile Diagrams: Reactant, Product, ∆H, Activation Energy, Slow Step
The reaction-energy diagram for a two step endothermic reaction with a ... The reaction-energy diagram for a two step endothermic reaction with a rate-limiting second step is to be drawn. Concept introduction: The graphical representation of chemical reaction in which x-axis represents energy of the reaction and y-axis represents the extent of reaction process is called energy profile diagram.
7.4 SN1 Reaction Mechanisms, Energy Diagram and Stereochemistry Figure 7.4a Energy diagram for SN1 reaction between (CH3)3CBr and H2O. The connection between the first two curves represent the carbocation intermediate. Generally, the intermediate is the product of one step of a reaction and the reactant for the next step. The intermediate is at a relatively lower energy level compared to the transition ...












![Solved] Select the Potential Energy Diagram That Represents a ...](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3da2_606e_9180_bb23921d32b1_TB5902_00.jpg)





/chapter9/pages3and4/page3and4_files/addition_energy_diagram.png)



Post a Comment for "40 energy diagram for a two step reaction"